Preparation of Gels
Preparation of a Gel as a Colloid:
Agar-agar is extracted from marine agarophytes algae. Agar is a dried hydrophilic, colloidal substance. Chemically it is a complex mixture of polysaccharides composed of two major fractions – agarose and agaropectin units. An agarose chain is a neutral alternating copolymer of β1,3-linked D-galactose and α-1,4-linked 3,6-anhydro-L-galactose (Figure 1). Normally agarose represents about two-thirds of the natural agar-agar. Agarose is the gelling fraction. Agaropectin is composed of agarose substituted at irregular intervals with sulphate esters, methyl esters, and/or pyruvate residues. It probably exists in the form of calcium salt or a mixture of calcium and magnesium salts.
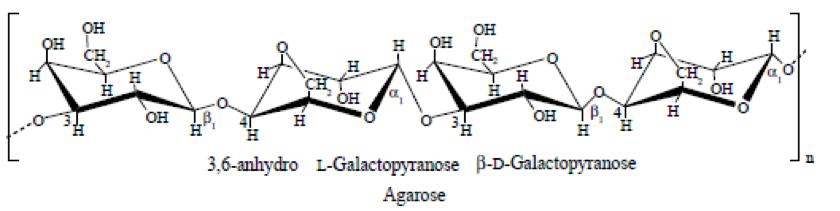
Fig1. Structure of an agarose polymer.
The solubility as well as the gel-forming ability of agar polysaccharides depends on the relative hydrophobicity of the basic repeating unit, the alternating 1,3-linked β-β-galactopyranose and 1,4-linked 3,6-anhydro-α-L-galactopyranose and its substitution by hydrophobic (methoxyl) and polar (sulphate, pyruvate) groups. Although agar-agar is insoluble in cold water, it can absorb about 20 times its own weight of water and swells considerably. It dissolves readily in boiling water. Gelling occurs on cooling the hot solution of agar-agar. Gel formation takes place for even only 0.50% agar-agar solutions at temperatures between ~30°C and 45°C depending on the species of algae used for its extraction. The agar gel is called ‘physical gel’ due to its thermo-reversible nature. Agar gels can be melted by heating and set to gels again on cooling. The gel is melted by heating to 85 – 95°C. This wide gap between the gelling and gel melting temperatures or very high gelling hysteresis of agar-agar makes it suitable for many food applications.
The agarose chain forms anti-symmetric double helices on cooling that form clumps of helices due to hydrogen bonding. The double-helical form of agarose has been proposed to be an intermediate in gelation. Three equatorial hydrogen atoms on the 3,6-anhydro-L-galactose residues, which constrain the molecule to form a helix, give rise to the gelling property of agar-agar. The interaction of the helices causes the formation of the gel. Exterior hydroxyl groups cause aggregation of up to 10,000 of these helices forming suprafibers. The fibre bundles and microgel domains are held together by hydrogen bonds. These helices interact and grow into still larger structural units, eventually forming a three-dimensional continuous network spanning the entire volume of the liquid. The network structure results in the interstitial pockets of 50 – 200 nm diameters. The liquid is entrapped in the interstitial pockets of the network. Though gels are mostly liquid both by volume and weight, yet they do not flow when in the steady-state and exhibit solid-like behavior due to the three-dimensional cross-linked network within the liquid. Thermodynamically, the process involves a balance between particle-particle interactions and the particle-solvent interactions in solution. The loss of conformational freedom and hydration energy on gelation is compensated by energetically favorable chain-chain interactions. Agar gel and some other gels melt to liquid states if they are reheated. Because the forces (like configurational entropy) favoring the amorphous state outweigh those (like hydrogen bonds) favoring the aggregated state.
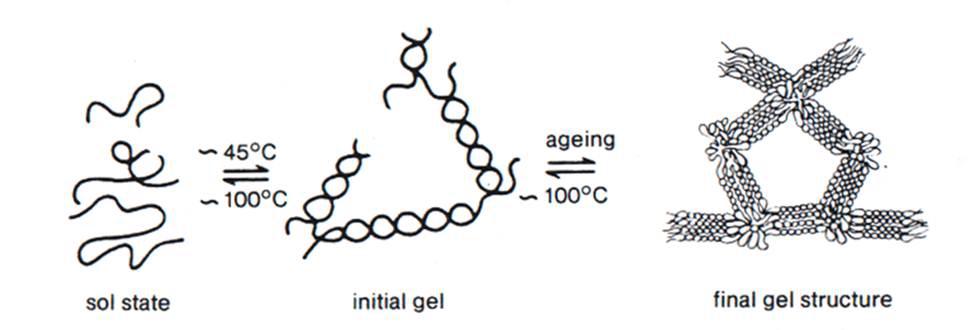
Fig2. Development of network structure in agarose gel. (Courtesy: T Laas, Doctoral Thesis, Acta Universitatis Upsaliensis, 1975).
The degree of hydration plays an important role in the stability of agar-agar gel. In solution, agar-agar is negatively charged. This charge also plays an equally important role in its stability. In addition to the concentration, the strength of the agar-agar gel depends on the pH, sugar content, rate of gelation, etc. Lower agar-agar concentration produces more supple and fragile gel, whereas more firm and brittle gel forms at higher concentrations. An increasing quantity of sugar gives harder gels with less cohesive texture. Gel strength decreases with the decrease in the pH of the solution. One should avoid exposing agar-agar solutions to high temperatures or to pHs lower than 6.0 for longer periods of time. Both the factors can degrade solutions of agar-agar, which may result in lower gel strength after the gel formation. Atomic force microscope (AFM) images of “unperturbed” gel showed a strong dependence of interstitial pore size and its distribution on the ionic strength of the solution, e.g., the concentration of Tris-borate-EDTA (TBE) buffer. For a given ionic strength, the pore size decreases, and the pore size distribution narrows with the increase in agarose concentration.
Agar gel is used in Cell Structure and Microbiology, Molecular Biology, and Biochemistry for electrophoresis, culture media, immunodiffusion, gel chromatography, support for biocatalysis, growth of protein crystals, etc. Agar-agar, being of vegetable origin, is an ideal substitute for gelatin, an animal product, especially for its use in food products.
